Background - Plants and Climate

That a relationship exists between vegetation and climate has long been recognized and is easy to see. Anyone who has travelled extensively is aware that the overall appearance of vegetation (i.e. its physiognomy), but not necessarily its species composition, tends to be characteristic of the particular climatic regime in which the vegetation grows. Vegetation can therefore be classified physiognomically, and climatic signals can be derived from it independent of taxonomic considerations (e.g. Holdridge, 1947). This is because many plant morphologies represent particular engineering solutions to particular environmental constraints. Only those plants with a genome that affords certain appropriate phenotypic expressions are able to survive in particular climatic regimes. Inappropriate phenotypes fail to survive, either through direct environmental elimination (the physical environment takes its toll directly), or by competition with better suited plants (eventually leading to the demise of the less well suited, and therefore less competitively successful, individuals).
Although all parts of a plant throughout all stages of the life cycle contribute to the overall success or failure of the individual, the organ that plays the most critical role in environmental adaptation is the leaf. A leaf is an integrated system of architectural and chemical systems in which a change in one component has to be accompanied by changes to other components if the integrity of the whole is to be maintained. The photosynthetic function of a leaf demands that it is efficient at intercepting light and exchanging gases with the atmosphere, while affording the minimum water loss concomitant with maintaining water flow within the plant and evaporative cooling of the leaf surface. All this must be achieved with the minimum of structural tissue investment because building leaf tissue costs energy and food resources. Thus there are only a limited range of "engineering solutions" that can satisfy, approximately, the often conflicting constraints that exist in a given set of environmental conditions. Because the unchanging laws of gas diffusion, fluid flow, and mechanics impose these constraints, these solutions are time stable and independent of taxonomic affinity (Spicer, 1999).
So pervasive is the premium on selecting for leaf efficiency that leaf physiognomic "tuning" to environment can be seen to vary not only between plant lineages, and between individual plants within lineages (ecophenotypes), but also within individual plants in time and space. For example, leaves at the top of a tree crown are exposed to high light levels and wind energies and tend to be small, thick sun leaves. In contrast, those in the darker, more sheltered, humid subcanopy space tend to be larger and thinner (shade leaves). If the environment around a tree changes with time, for example, through removal of surrounding trees, then subsequent cohorts of leaves will display different appropriate morphologies.
The capability for leaf morphological plasticity must be genetically coded, but how independent of genetic predetermination are character states that might have some correlation with climate? At first thought, leaf size, because of its variability on even a single individual plant, or variability during different growth stages of the plant, might be thought to be subject to minimal genetic predetermination, but this cannot be valid. Plants must produce leaves that are adaptive to the entire growing season, and this production must, therefore, be genetically predetermined. Plants in the Mediterranean region, California and Chile, for example, receive abundant rainfall during the spring when the new leaf crop is produced, followed by extreme summer drought. If the plants responded only to the spring rains and produced large leaves these leaves would be non-adaptive during the summertime. Clearly selection favours genotypes that are tuned to the overall climatic conditions and not just those experienced by the plant at leaf development. Vegetation in recently glaciated and non-glaciated parts of the Northern Hemisphere also demonstrates a good correlation with climate. This shows that genetic, and phenotypic, tuning of foliar physiognomy takes place over geologically short timescales (< 1 million years) as a result of taxonomic elimination and migration as well as selection for novel genotypes that arise as the result of chance mutation or hybridization.
Leaf form and phenotypic variation involves a complex interplay between the genome and developmental programmes. Often single genes can influence the forms of several characters (Pigliucci, 2003) and there is inherent flexibility in leaf developmental programmes (Falconer & Mackay, 1996; Juenger et al., 2005; Rodriguez et al., 2014). Unsurprisingly then, in terms of the CLAMP character set and scoring protocols, almost all characters are correlated either positively or negatively as shown in the diagram below. Changes in one character have to result in changes to other characters otherwise "tuning" to the environment will be compromised. By examining many characters simultaneously, as is done in CLAMP, errors in palaeoclimate retrodiction that might arise from single character change are reduced.
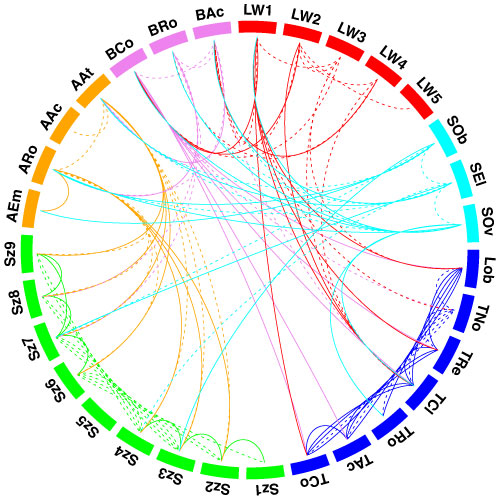 |
| Diagram illustrating the relationships between CLAMP leaf characters shown by pairwise Pearson correlation coefficients. The 31 leaf characters used in CLAMP are grouped: lobing and tooth form, leaf size, apex form, base form, length-to-width ratio and shape. Leaf character groups are indicated by different colours: red – length-to-width ratios, light blue – leaf shape, dark blue – margin characters, green – leaf size, orange – apex characters, pink – base characters. The solid lines represent values of pairwise Pearson correlation above ≤ 0.5 (a significant positive correlation), while the dashed lines indicate correlations ≥ -0.5 (a significant negative correlation). Some correlations are trivial in that they arise from the scoring regime (e.g., “no teeth” is negatively correlated with all tooth characters because leaves without teeth will not be scored for tooth characters) and are shown as links within leaf character groups. Meaningful correlations are those that link different character groups. For example the cordate base condition is positively correlated with several tooth characters. Abbreviations are as follows: Lob: Lobing; TNo: No Teeth; TRe: Regularity of tooth spacing; TCl: Closeness of teeth; TRo: Teeth rounded and (or) appressed; TAc: Teeth acute; TCo: Teeth compound; Sz1: Leaf Size, Nanophyll; Sz2: Leaf Size ,Leptophyll 1; Sz3: Leaf Size, Leptophyll 2; Sz4: Leaf Size ,Micro 1; Sz5: Leaf Size, Micro 2; Sz6: Leaf Size, Micro 3; Sz7: Leaf Size, Mesophyll 1; Sz8: Leaf Size, Mesophyll 2; Sz9: Leaf Size, Mesophyll 3; AEm: Apex emarginate; ARo: Apex round; AAc: Apex acute; AAt: Apex attenuate; BCo: Base cordate; Bro: Base round; BAc: Base acute; LW1: Length to width less than 1:1; LW2: Length to width 1-2:1; LW3: Length to width 2-3:1; LW4: Length to width 3-4:1; LW5: Length to width greater than 4:1; Sob: Shape obovate; SEl: Shape elliptic; SOv: Shape ovate. See Yang et al. (2015) for further details. |

